New Catalyst is Three Times Better at Splitting Water
Results Suggest a More Efficient Way to Convert Solar and Wind Power to Renewable Fuels
With a combination of theory and clever, meticulous gel-making, scientists from the Department of Energy’s SLAC National Accelerator Laboratory and the University of Toronto have developed a new type of catalyst that’s three times better than the previous record-holder at splitting water into hydrogen and oxygen – the vital first step in making fuels from renewable solar and wind power.
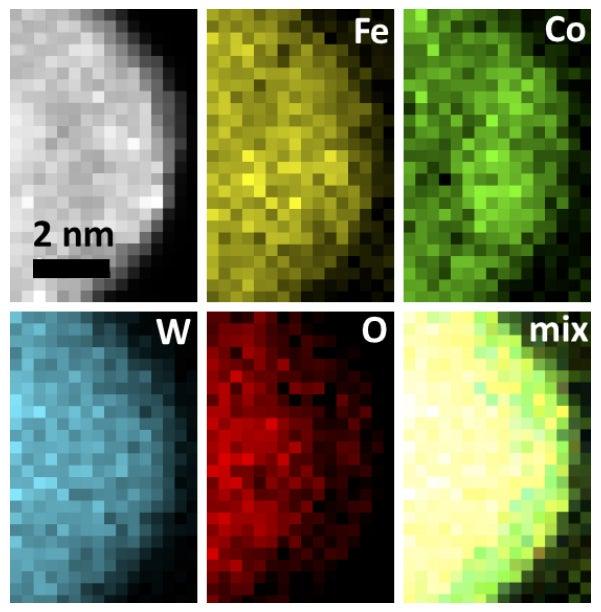
The research, published today in the journal Science, outlines a potential way to make a future generation of water-splitting catalysts from three abundant metals – iron, cobalt and tungsten – rather than the rare, costly metals that many of today’s catalysts rely on.
“The good things about this catalyst are that it’s easy to make, its production can be very easily scaled up without any super-advanced tools, it’s consistent, and it’s very robust,” said Aleksandra Vojvodic, a SLAC staff scientist with the SUNCAT Center for Interface Science and Catalysis who led the theoretical side of the work.
Storing Sun and Wind Power
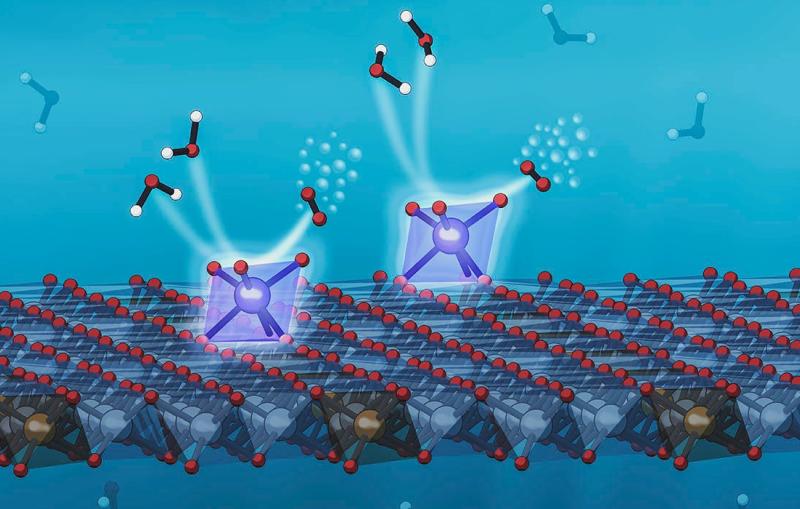
Scientists have been searching for an efficient way to store electricity generated by solar and wind power so it can be used any time – not just when the sun shines and breezes blow. One way to do that is to use the electrical current to split water molecules into hydrogen and oxygen, and store the hydrogen to use later as fuel.
This reaction takes place in several steps, each requiring a catalyst – a substance that promotes chemical reactions without being consumed itself – to move it briskly along. In this case the scientists focused on a step where oxygen atoms pair up to form a gas that bubbles away, which has been a bottleneck in the process.
In previous work, Vojvodic and her SUNCAT colleagues had used theory and computation to look at water-splitting oxide catalysts that contain one or two metals and predict ways to make them more active. For this study, Edward H. Sargent, a professor of electrical and computer engineering at the University of Toronto, asked them to look at the effect of adding tungsten – a heavy, dense metal used in light bulb filaments and radiation shielding – to an iron-cobalt catalyst that worked, but not very efficiently.
With the aid of powerful computers at SLAC and elsewhere and state-of-the-art computational tools, the SUNCAT team determined that adding tungsten should dramatically increase the catalyst’s activity – especially if the three metals could be mixed so thoroughly that their atoms were uniformly distributed near the active site of the catalyst, where the reaction takes place, rather than separating into individual clusters as they normally tend to do.
“Tungsten is quite a large atom compared to the other two, and when you add a little bit of it, it expands the atomic lattice, and this affects the reaction not only geometrically but also electronically,” Vojvodic said. “We were able to understand, on the atomic scale, why it works, and then that was verified experimentally.”
Add Metal Atoms, Mix and Gel
Based on that information, Sargent’s team developed a novel way to distribute the three metals uniformly within the catalyst: They dissolved the metals and other ingredients in a solution and then slowly turned the solution into a gel at room temperature, tweaking the process so the metal atoms did not clump together. The gel was then dried into a white powder whose particles were riddled with tiny pores, increasing the surface area where chemicals can attach and react with each other.
In tests, the catalyst was able to generate oxygen gas three times faster, per unit weight, than the previous record-holder, Sargent said, and it also proved to be stable through hundreds of reaction cycles.
“It’s a big advance, although there’s still more room to improve,” he said. “"And we will need to make catalysts and electrolysis systems even more efficient, cost effective and high intensity in their operation in order to drive down the cost of producing renewable hydrogen fuels to an even more competitive level.”
Sargent said the researchers hope to use the same method to develop other three-metal catalysts for splitting water and also for splitting carbon dioxide, a greenhouse gas released by burning fossil fuels, to make renewable fuels and chemical feed stocks. He and five other members of the University of Toronto team have filed for a provisional patent on the technique for preparing the catalyst.
Future Directions
“There are a lot of things we further need to understand,” Vojvodic said. “Are there other abundant metals we can test as mixtures in oxides? What are the optimal mixtures of the components? How stable is the catalyst, and how can we scale up its production? It needs to be tested at the device level, really.”
Jeffrey C. Grossman, a professor of materials science and engineering at MIT who was not involved in the study, said, “The work impressively highlights the power of tightly coupled computational materials science with advanced experimental techniques, and sets a high bar for such a combined approach. It opens new avenues to speed progress in efficient materials for energy conversion and storage."
SLAC research associate Michal Bajdich and Stanford postdoctoral researcher Max García-Melchor also contributed to this work, along with researchers from the DOE’s Brookhaven National Laboratory; East China University of Science & Technology, Tianjin University and the Beijing Synchrotron Radiation Facility in China; and the Canadian Light Source. The research was funded by a number of sources, including the Ontario Research Fund – Research Excellence Program, Natural Sciences and Engineering Research Council of Canada and the CIFAR Bio-Inspired Solar Energy Program, as well as the DOE Office of Science, which funds SUNCAT, and the SLAC Laboratory Directed Research and Development program.
Citation: B. Zhang et al., Science, 24 March 2016 (10.1126/science.aaf1525)
For questions or comments, contact the SLAC Office of Communications at communications@slac.stanford.edu.
SLAC is a multi-program laboratory exploring frontier questions in photon science, astrophysics, particle physics and accelerator research. Located in Menlo Park, Calif., SLAC is operated by Stanford University for the U.S. Department of Energy's Office of Science.
SLAC National Accelerator Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.